VenusP-Valve impresses at OCC-WCC 2024: Eight-year follow-up data affirms long-term safety and efficacy
Hangzhou, China, June 30, 2024 -- At the Structural Heart Disease Forum of the 18th Oriental Congress of Cardiology Together with the World Congress of Cardiology (OCC-WCC 2024), Prof. Zhou Daxin from Zhongshan Hospital affiliated to Fudan University, presented the eight-year follow-up data from the pivotal clinical trial of VenusP-Valve, China's first transcatheter pulmonic valve replacement (TPVR) device.
SUMMARY
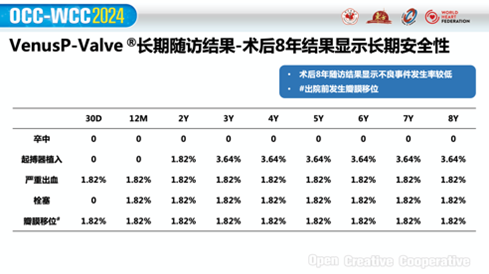
Long-term Safety: No increase in cardiac deaths at eight-year compared to one, three, five, and seven years post-procedure;
Long-term Efficacy: Good valve function, improved hemodynamics, and sustained patient benefits backed by eight-year post-procedural results;
Improved Quality of Life: Six female subjects became mothers during the follow-up period.

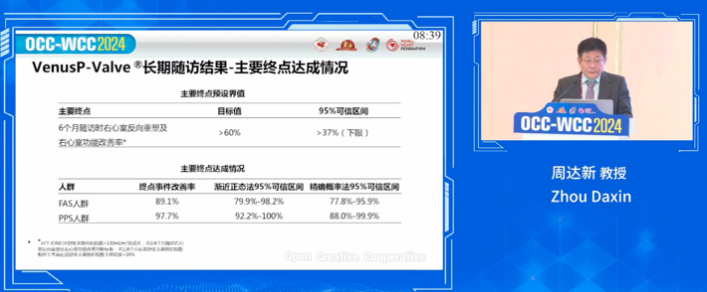
As noted by Prof. Zhou, the long-term follow-up results indicate a primary endpoint achievement rate of 97.7% for VenusP-Valve, with significant improvements in patients' right ventricular function and hemodynamics. The follow-up data reinforce the device's long-term safety and efficacy.
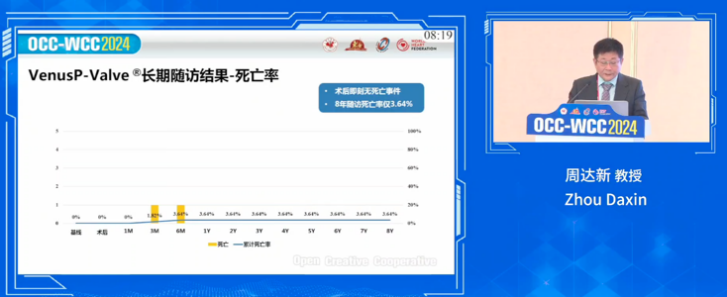
No immediate deaths were reported post-procedure. Mortality rate at eight years was only 3.64%, which remained consistent with the rates at one, three, five, and seven years post-procedure. In addition, the longest-followed patient has received telephone follow-up for 11 years.
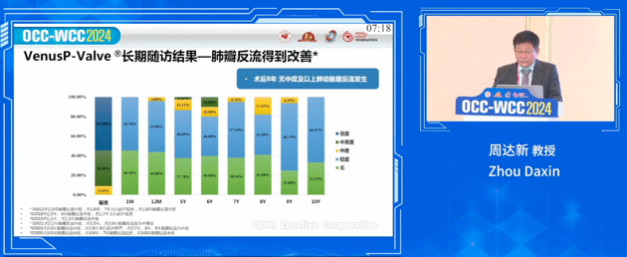
At eight years post-procedure, patients exhibited significant improvements in pulmonary regurgitation: severe pulmonary regurgitation decreased from 54.5% at baseline to 0%, and moderate-to-severe pulmonary regurgitation decreased from 36.4% at baseline to 0%. Furthermore, no cases of moderate or severe pulmonary regurgitation were observed at eight years.
The long-term follow-up results for VenusP-Valve also indicate that patients' left ventricular ejection fraction (LVEF) scores remained within the normal range and stable at eight years, and the mean pressure gradient (PGmean) also remained within the normal range and stable post-procedure. Moreover, the results found a low incidence of adverse events. During the follow-up period, five cases of endocarditis were reported, with two resulting from dental infections.
VenusP-Valve’s China pivotal clinical trial is a prospective, multi-center, single-arm clinical study designed to evaluate the feasibility, safety, and efficacy of percutaneous transcatheter pulmonic valve replacement in patients with severe pulmonary regurgitation following surgical correction for congenital heart defects with right ventricular outflow tract (RVOT) stenosis. The primary endpoint of the trial is right ventricular reverse remodeling and improvement in right ventricular function at six months post-procedure, defined by the right ventricular end-diastolic volume index (RVEDVI) measured by CMR being within the normal range (RVEDVI ≤ 108 mL/m²).
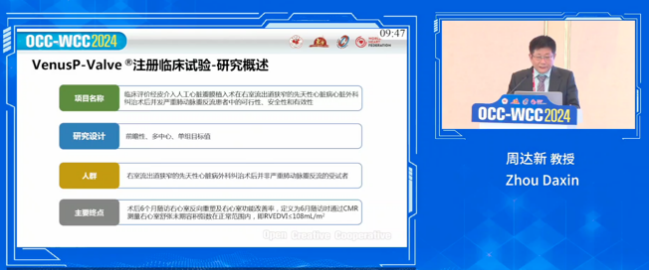
The trial enrolled 55 patients across six centers, i.e. Zhongshan Hospital affiliated to Fudan University, Shanghai Chest Hospital affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai Children's Medical Center affiliated to Shanghai Jiao Tong University School of Medicine, Fuwai Hospital of the Chinese Academy of Medical Sciences, West China Hospital of Sichuan University, and Xijing Hospital of Air Force Military Medical University. The longest patient follow-up period has reached 11 years.
Following its first clinical implantation in 2013, VenusP-Valve has been applied in clinical practice for 11 years. To date, the device has been included in national health insurance programs in Germany, France, etc., and has been approved in more than fifty countries, including China, Germany, France, the United Kingdom, Italy, Spain, Canada, and Australia, with its implantation seeing continuous growth in new hospitals and centers.
Earlier in 2022, VenusP-Valve received CE marking in the EU and approval from China's NMPA. In June 2024, VenusP-Valve completed its first implantation in the PROTEUS IDE Pivotal Clinical Study at the University of Virginia School of Medicine.
About Venus Medtech
Venus Medtech (Hangzhou) Inc. (02500.HK) is committed to structural heart innovation. We are developing and commercializing comprehensive solutions for structural heart disease. Our robust pipeline, encompassing all four heart valves from TAVR, TPVR, TMVR, and TTVR to hypertensive renal denervation (RDN) therapy, underscores our unwavering commitment.
For more information, please visit https://www.mercenarygeneral.com
SOURCE: Venus Medtech (Hangzhou) Inc.
*Provided for informational and academic purposes only, this content is not intended as professional medical or legal advice. Venus Medtech makes no representations, warranties or guarantees regarding the completeness, accuracy, or timeliness of this content.
*Venus Medtech makes no representations, warranties or guarantees regarding the property or clinical performance of any medical devices mentioned.
*VENUSMEDTECH, the stylized QI logo, VenusP-Valve, etc. are trademarks of Venus Medtech (Hangzhou) Inc.
Copyright 2024. Venus Medtech (Hangzhou) Inc. All Rights Reserved.
Prev:Venus Medtech VenusP-Valve Completed First Implantation in IDE Pivotal Clinical Study in U···
Next:Venus Medtech Named to the Inaugural High-Value Patent Cultivation Selection of Zhejiang P···